|

Review: Iridium-based catalysts look set to boost
efficiency of green hydrogen production
March 30, 2023
By Tsinghua
University Press
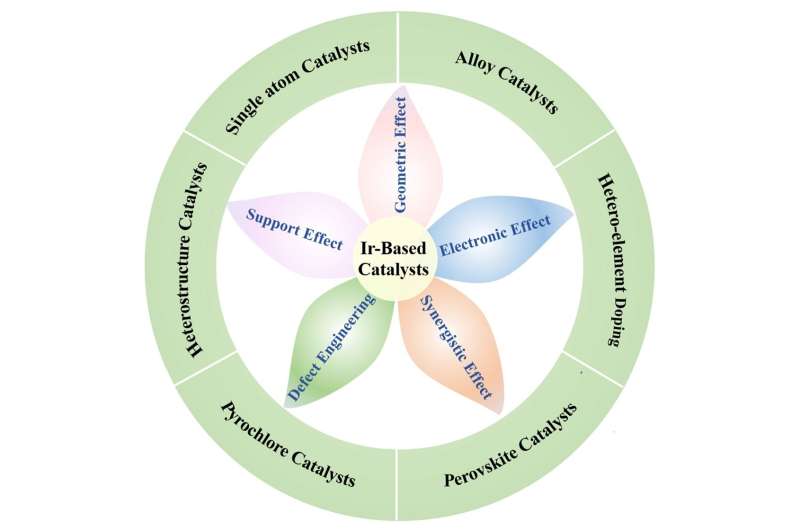
The graphic illustrates five different effects of Ir-based
catalysts, which were studied for future improvements
Hydrogen production powered by wind and solar energy is still too
expensive if it is to play a role in the clean transition via energy
storage and to help decarbonize hard-to-electrify sectors. Much effort
in reducing its cost focuses on enhancing production efficiency by
improving the performance of iridium-based catalysts that can speed up
the oxygen-related part of the electrochemical reaction involved in
splitting water into its component parts, hydrogen and oxygen.
A new review of the state of the field discusses its recent progress
and challenges and identifies research gaps that need to be filled
before such catalysts can achieve commercial viability.
The review paper was published in the journal Nano
Research Energy.
Cleanly produced hydrogen is
essential in the transition away from fossil fuels in order to avoid
dangerous climate change, both as an energy carrier to be used on its
own or as a component of a synthetic fuel for those sections of the
economy such as long-haul shipping and aviation that are hard to
electrify.
But such clean hydrogen
production—which is performed via electrolysis, using electricity
to split water into its component elements, hydrogen and oxygen—is
extremely energy intensive. This energy intensity of electrolysis in
turn makes clean production of hydrogen very expensive, and thus
uncompetitive with fossil fuels.
If this were not enough of a challenge, using wind and solar
energy as the source of clean electricity to power the
electrolysis—a form of hydrogen production termed 'green
hydrogen'—places a significant burden on the electrolyzers because
these energy sources are intermittent. The sun doesn't always shine
and the wind doesn't always blow.
This means that sometimes there is little to no current and at other
times, there can be a big spike of current, which places stress on the
electrolyzers, again pushing up costs. However, proton exchange
membrane water electrolyzers (PEMWE) are a very promising option here,
as PEMWEs can operate at high current densities such as those posed by
these spikes.
Electrolysis is a chemical reaction composed of two parts, or 'half
reactions.' One is the hydrogen evolution reaction (HER), which
generates the hydrogen, and the other is the oxygen evolution reaction
(OER), which produces the oxygen. But it is actually the latter
reaction that is most important with respect to the energy efficiency
of the overall process and thus production of clean hydrogen.
And so to reduce the energy demands and thus the cost of clean
production of hydrogen, a lot of research has focused on superior
catalysts—chemicals that speed up a chemical reaction—for the OER part
of the process and that pair well with PEMWEs.
However, the severe corrosion in the acidic environment of PEMWEs
makes most non-precious metal-based catalysts—for example using
cobalt, nickel, or iron—unstable. But iridium-based catalysts exhibit
much better catalytic stability in these harsh acidic conditions.
A number of recent studies have reported significant advances in the
development of iridium-based catalysts for green hydrogen production,
including the use of new synthesis methods and the optimization of catalyst structures
and compositions.
However, there are still several research challenges that need to be
addressed to fully realize the potential of iridium-based catalysts
for green hydrogen production. One major challenge is the high cost of
iridium—and high costs are precisely what novel catalysts were
intended to avoid.
"To overcome this, researchers are exploring new synthesis methods and
alternative catalyst materials that can replace iridium or reduce the
amount of iridium required," said Chunyun Wang, of the School of
Chemistry and Chemical Engineering at Yangzhou University and lead
author of the review. "Some novel and effective options have emerged
recently, such as iridium oxides, perovskites, pyrochlores, and
single-atom catalysts."
"And so we thought it was about time that we paused and assessed the
state of play in iridium-based catalysts for green hydrogen production
with a review paper," added Alex Schechter, a chemist with Ariel
University in Israel and co-author of the review paper. "The benefit
of this is to pool information across many different teams of
researchers and, crucially, identify research gaps."
The review focuses in particular on how the catalysis operates (the
catalytic mechanism), design of catalysts, and strategies for
synthesis of catalysts. In particular, the analysis looks at different
attributes of catalysts that affect their promotion of the catalysis
process including geometric effects, electronic effects, synergistic
effects, defect engineering and support effects, and how different
research teams have dealt with each option to try to improve
performance.
Geometric effects in essence describe the shape, structure and size of
the catalyst molecule, including which of its crystal planes are
exposed, and how atomic arrangements might be ordered or disordered.
All of this significantly affects catalyst performance. Electronic
effects refer to the structure of electrons associated with the
relevant molecules.
Synergistic effects are those where two or more ingredients come
together to produce a superior result than either one on its own.
Defect engineering involves efforts to design the surface chemistry of
catalysts via voids, dislocations, vacancies and so on—deliberately
introducing imperfections—so as to increase the number of places where
the chemical reaction can take place (active sites). And support
effects come from metals that interact with and support the catalyst.
The reviewers concluded after surveying their field that the most
successful strategy for improving the performance of iridium-based
catalysts includes defect engineering, adjusting synergistic effects
and altering geometric effects. The number of exposed active sites can
be increased by constructing a porous structure and introducing
supports for the catalyst that promote transfer of both mass and
electrons. And enhanced metal-support interaction can increase the
long-term stability of the catalysts.
Despite the considerable research success, the field still faces
challenges. Many high-performance iridium-based catalysts have been
developed, but most of them can only be synthesized on a small scale
of just a few grams or even hundreds of milligrams in the laboratory.
Complex preparation processes thus must be simplified.
In addition, lab conditions are a bit too ideal compared to actual
catalytic systems, and so real-world conditions need to be part of any
follow-up research. This includes looking at realistic electrolyzer
temperature, current density, and product delivery, amongst other
aspects, that will enable evaluation of performance catalysts in
practical applications.
And beyond the catalysts themselves, other components need to be
optimized as well, including the development of electrode plates with
high corrosion resistance and low cost, proton exchange membranes with
high proton transport capacity.
The reviewers stressed however that none of these challenges are
deal-breakers for iridium-based catalysts for green hydrogen
production. Instead these represent possible avenues for new research
that may deliver the breakthroughs this process requires to achieve
commercial viability.
Green Play Ammonia™, Yielder® NFuel Energy.
Spokane, Washington. 99212
www.exactrix.com
509 995 1879 cell, Pacific.
exactrix@exactrix.com
|