
UW Madison chemists discover new way to harness
energy from ammonia
12 November 2021
A
research team at the University of Wisconsin–Madison has identified a
new way to convert ammonia to nitrogen gas through a process that
could be a step toward ammonia replacing carbon-based fuels. The
discovery of this technique, which uses a metal catalyst and
releases—rather than requires—energy, was reported in Nature
Chemistry and has received a provisional patent from the Wisconsin
Alumni Research Foundation.
The electrochemical conversion of ammonia to dinitrogen in a direct
ammonia fuel cell (DAFC) is a necessary technology for the realization
of a nitrogen economy. Previous efforts to catalyse this reaction with
molecular complexes required the addition of exogenous oxidizing
reagents or application of potentials greater than the thermodynamic
potential for the oxygen reduction reaction—the cathodic process of a
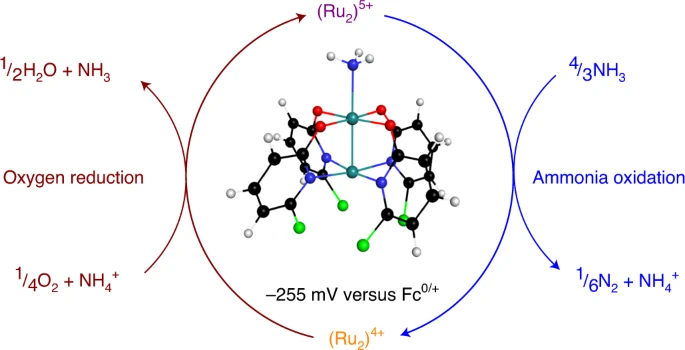
DAFC. We report a stable metal–metal bonded diruthenium complex that
spontaneously produces dinitrogen from ammonia under ambient
conditions. The resulting reduced diruthenium material can be
reoxidized with oxygen for subsequent reactions with ammonia,
demonstrating its ability to spontaneously promote both half-reactions
necessary for a DAFC.
The
scientists found that the addition of ammonia to a metal catalyst
containing the ruthenium spontaneously produced nitrogen; no added
energy was required. This process can be harnessed to produce
electricity, with protons and nitrogen gas as byproducts. In addition,
the metal complex can be recycled through exposure to oxygen and used
repeatedly.
We
figured out that, not only are we making nitrogen, we are making it
under conditions that are completely unprecedented. To be able to
complete the ammonia-to-nitrogen reaction under ambient conditions—and
get energy—is a pretty big deal.
—John
Berry, corresponding author
Ammonia has been burned as a fuel source for many years. During World
War II, it was used in automobiles, and scientists today are
considering ways to burn it in engines as a replacement for gasoline,
particularly in the maritime industry. However, burning ammonia
releases toxic nitrogen oxide gases.
The
new reaction avoids those toxic byproducts. If the reaction were
housed in a fuel cell where ammonia and ruthenium react at an
electrode surface, it could cleanly produce electricity without the
need for a catalytic converter.
For a fuel cell, we want an electrical output, not input. We
discovered chemical compounds that catalyze the conversion of ammonia
to nitrogen at room temperature, without any applied voltage or added
chemicals. This is the first process, as far as we know, to do that.
—Christian Wallen, co-author
We
have an established infrastructure for distribution of ammonia, which
is already mass produced from nitrogen and hydrogen in the Haber-Bosch
process. This technology could enable a carbon-free fuel economy, but
it’s one half of the puzzle. One of the drawbacks of ammonia synthesis
is that the hydrogen we use to make ammonia comes from natural gas and
fossil fuels.
—Michael Trenerry, lead author
This
is changing, however, as ammonia producers attempt to produce “green”
ammonia, in which the hydrogen atoms are supplied by carbon-neutral
water electrolysis instead of the energy-intensive Haber-Bosch
process.
As
the ammonia synthesis challenges are met, according to Berry, there
will be many benefits to using ammonia as a common energy source or
fuel. It’s compressible, like propane, easy to transport and easy to
store. Though some ammonia fuel cells already exist, they, unlike this
new process, require added energy, for example, by first splitting
ammonia into nitrogen and hydrogen.
The
group’s next steps include figuring out how to engineer a fuel cell
that takes advantage of the new discovery and considering
environmentally friendly ways to create the needed starting materials.
This
work was supported by the US Department of Energy (DOE).
Resources
-
Trenerry, M.J., Wallen, C.M.,
Brown, T.R. et al. “Spontaneous N2 formation
by a diruthenium complex enables electrocatalytic and aerobic
oxidation of ammonia.” Nat. Chem. doi: 10.1038/s41557-021-00797-w
Posted on 12 November 2021 in Ammonia, Catalysts, Fuel
Cells, Market
Background | Permalink | Comments
Green Play Ammonia™, Yielder® NFuel Energy.
Spokane, Washington. 99212
www.exactrix.com
509 995 1879 cell, Pacific.
exactrix@exactrix.com
|